Std 10 All important chemical Reactions to revise quickly
10th Science 2022 : Most Important Chemical Reactions To Revise Before Exam
Check chapter-wise important chemical reactions of Class 10th Science. The list of chemical reactions available here is from Class 10th Science NCERT textbooks, previous years' papers and the latest sample paper.
Science is one of the important subjects of Class 10th and students preparing for this subject often search for chemical reactions important for the Class 10 Science Board Exam. In this article, you will get important chemical reactions, resources (such as Important Questions, Sample Papers, Paper Pattern, etc.) and a few useful tips for exam preparation.
Chapter-wise Important Chemical Reactions for Class 10 Science Board Exam are given below:
Chapter 1
Chemical Reactions and Equations
• Mg + O2 → 2MgO
• Zn + H2SO4→ ZnSO4 + H2
• 3Fe + 4H2O → Fe3O4 + 4H2
• CaO (s) + H2O (l) → Ca (OH)2 (aq)
(Quick lime) (Slaked lime)
• C(s) + O2 (g) → CO2 (g)
• 2 H2 (g) + O2 (g) → 2H2O (l)
• CH4 (g) + 2 O2 (g) → CO2 (g) + 2H2O (g)
• C6H12O6 (aq) + 6O2 (aq) → 6 CO2 (aq) + 6 H2O (l) + energy
(Glucose) (Oxygen)
• 2FeSO4(s) → Fe2O3 (s) + SO2 (g) + SO3 (g)
(Ferrous sulphate) (Ferric oxide)
• CaCO3(s) + Heat → CaO (s) + CO2 (g)
(Limestone) (Quick lime)
• 2Pb (NO3)2 (s) + Heat → 2PbO(s) + 4NO2 (g) + O2 (g)
(Lead nitrate) (Lead oxide) (Nitrogen dioxide)
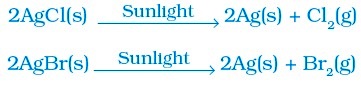
• Na2SO4 (aq) + BaCl2 (aq) → BaSO4 (s) + 2NaCl (aq)
• ZnO + C →Zn + CO
• MnO2 + HCl →MnCl 2 + 2H2O + Cl2
Chapter 2
Acids, Bases and Salts
• Reaction of acids and bases with metals:
Acid + Metal → Salt + Hydrogen gas
Example:
2 NaOH + Zn → Na2ZnO2 + H2
(Sodium zincate)
• Reaction of base and acid to give salt (neutralisation reaction):
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Other reactions:
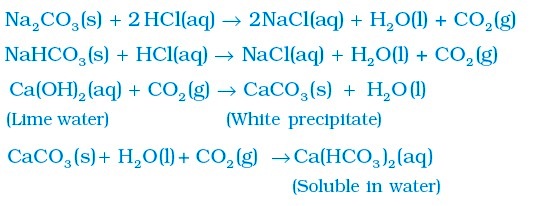
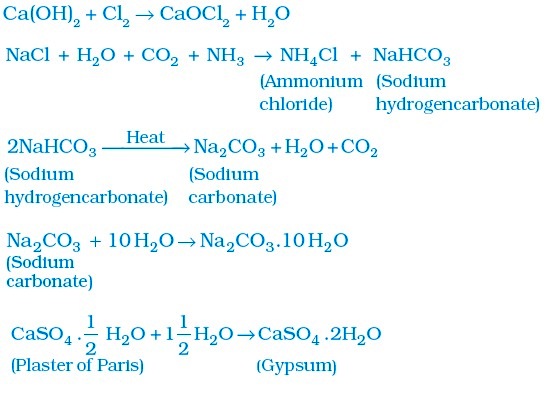
Chapter 3
Metals and Non-metals
• Chemical properties of metals:
Metal + Oxygen → Metal oxide
Example:
• 2Cu + O2 → 2CuO
• 4Al + 3O2 → 2Al2O3
Aluminum oxide reacts in the following manner with acids and bases:
• Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
• Al2O3 + 2 NaOH → 2 NaAlO2 + H2O
Sodium oxide and potassium oxide dissolve in water to produce alkalis:
• Na2O(s) + H2O (l) → 2 NaOH (aq)
• K2O(s) + H2O (l) → 2 KOH (aq)
Reactions of Metal with water
Metal + Water → Metal oxide + Hydrogen
Metal oxide + Water → Metal hydroxide
Example:
• 2K(s) + 2H2O (l) → 2KOH (aq) + H2 (g) + heat energy
• 2Na(s) + 2H2O (l) → 2 NaOH (aq) + H2 (g) + heat energy
• Ca(s) + 2 H2O (l) → Ca (OH)2 (aq) + H2(g)
• 2 Al (s) + 3H2O (g) → Al2O3 (s) + 3H2 (g)
• 3 Fe (s) + 4H2O (g) → Fe3O4(s) + 4H2 (g)
Check formation of NaCl and its electron dot structure form NCERT Textbook.
Chemical reactions related to extraction of metal are also important for CBSE 10th Science Board Exam 2019:
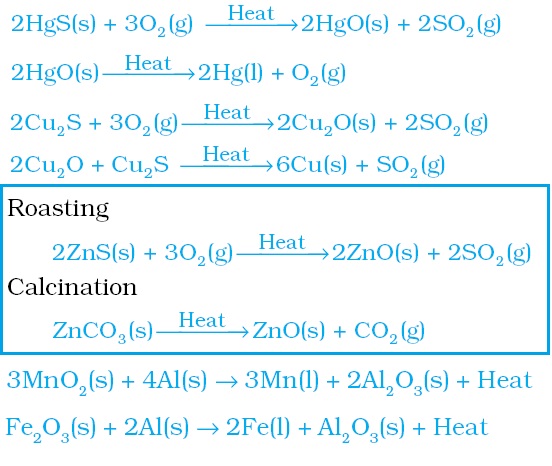
Chapter 4
Carbon and its Compounds
Chemical Properties of Carbon Compounds
Combustion of carbon and its compounds:
(i) C + O2 → CO2 + heat and light
(ii) CH4 + 2O2 → CO2 + 2H2O + heat and light
(iii) CH3CH2OH + 2O2 → 2CO2 + 3H2O + heat and light
Oxidation of carbon compounds:
Conversion of alcohol to carboxylic acid

Addition Reaction:
Hydrogenation of vegetable oils using a nickel catalyst:
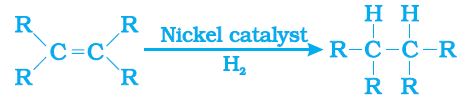
Properties of Ethanol:
Reactions of Ethanol with sodium –
2Na + 2CH3CH2OH → 2 CH3CH2O–Na+ + H2
(Sodium ethoxide)
Heating of ethanol:
Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene –

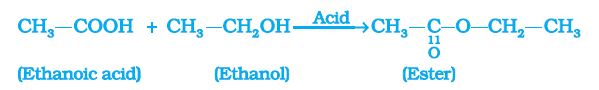
Esterification reaction:
Esters (sweet-smelling substances used in making perfumes and as flavouring agents) are most commonly formed by reaction of an acid and an alcohol. Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester –
Saponification:
Esters react in the presence of an acid or a base to give back the alcohol and carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap.

Chapter 6
Life Processes

Process of photosynthesis

Chapter 14
Sources of Energy
Nuclear fusion: Most commonly hydrogen or hydrogen isotopes to create helium, such as
2H + 2H → 3He (+ n)
It releases a tremendous amount of energy
Chapter 15
Our Environment
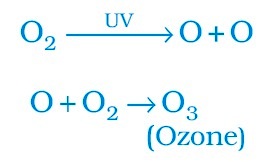
Formation of ozone:
Ozone at the higher levels of the atmosphere is a product of UV radiation acting on oxygen (O2) molecule. The higher energy UV radiations split apart some moleculer oxygen (O2) into free oxygen (O) atoms. These atoms then combine with the molecular oxygen to form ozone as shown
Comments
Post a Comment