Std 10 Important case study base questions
NCERT 2022 : Science Important Case-Based Questions with Solution
We provide you with the important exam stuff that has been put together by the subject experts who carry tremendous experience in the teaching field. In this article, we have provided below important case study questions for Ncert 10 Science.
These questions have been prepared specially for the Ncert based Exam, considering the latest pattern and reduced syllabus. Students must practice the chapter-wise important questions provided below to clearly understand the format of questions and increase their chances of scoring high in the Class 10 Science Exam 2022.
Chapter – 4
Carbon and its Compounds
Q. Read the following carefully.
In covalent compounds atoms share valence electrons to satisfy the octet. Each atom shares one pair or two pairs or three pairs of electrons depending on their combining capacity. In electron dot structures only number of valence electrons are shown around the symbols of constituent atoms. Carbon using its valency of four can make either single, double or triple bonds with other carbon atoms or any other atoms. Carbons self-linking property is called catenation. In hydrocarbons carbon makes aliphatic or cyclic molecules they are either saturated or unsaturated. Based on these facts Read the following paragraph and answer the questions given below. An element X combines with Y to form a colourless odourless gas, Z which turns lime water milky is the major constituent of all organic molecules. Five X atoms combines with hydrogens to form a cyclic saturated hydrocarbon J and aliphatic unsaturated hydrocarbon Q.Q is used in gas welding.
(a) Identify compound Z and draw its electron dot structure.
(b) Write the chemical formula and IUPAC name of compound Q
(c) What is the common name of Q
(d) How many single covalent bonds are present in compound J?
Answer:
a) Z is CO2 its electron dot structure is
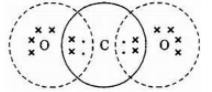
b) C2H2,ethyne c) Acetylene
c) Acetylene
d) 15
Q. Read the following and answer questions from (i) to (iv)
The compounds which have the same molecular formula but differ from each other in physical or chemical properties are called isomers and the phenomenon is called isomerism. When the isomerism is due to difference in the arrangement of atoms within the molecule, without any reference to space, the phenomenon is called structural isomerism. In other words. Structural isomers are compounds that have the same molecular formula but different structural formulas, i.e., they are different in the order in which different atoms are linked. In these compounds, carbon atoms can be linked together in the form of straight chains,branched chains or even rings.
(i) Which of the following sets of compounds have same molecular formula?
(a) Butane and iso-butane
(b) Cyclohexane and hexene
(c) Propanal and propanone
(d) All of these
(ii) In order to form branching, an organic compound must have a minimum of
(a) four carbon atoms
(b) three carbon atoms
(c) five carbon atoms
(d) any number of carbon atoms.
(iii) Which of the following is an isomeric pair?
(a) Ethane and propane
(b) Ethane and ethene
(c) Propane and butane
(d) Butane and 2-methylpropane
(iv) Among the following the one having longest chain is
(a) neo-pentane
(b) iso-pentane
(c) 2-methylpentane
(d) 2,2-dimethylbutane.
Answer:
(i) (d) All of these
(ii) (a) four carbon atoms
(iii) (d) Butane and 2-methylpropane
(iv) (c) 2-methylpentane
Chapter – 5
Periodic Classification of Elements
Q. Read the following carefully and answer the questions.
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral.
Based on the above information answer the following questions:
(1) To which group or period of the Periodic Table do the listed elements belong?
(2) What would be the nature of compound formed by a combination of elements B and F?
(3) Which two of these elements could definitely be metals?
(4) If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
Answer:
1. A and B belong to group 1 and 2 because they form basic oxides.
C belongs to group 13 as it has 3 valence electrons.
D belongs to group 14 as it forms almost neutral oxide.
E and F belong to group 15 and 16 as they form acidic oxides,
G belongs to group 17 as it has 7 valence electrons and H belongs to group 18.
They belong to 3rd period of the Periodic Table because AG is NaCl, added in a small amount to almost all vegetable dishes during cooking and Na and Cl belong to 3rd period.
2. Ionic compounds will be formed because ‘B’ is metal and ‘F’ is non-metal. ‘B’ can lose two electrons and ‘F’ can gain two electrons.
3. A and B are definitely metals as they form basic oxides.
4. CG3 is the formula of the compound formed by combination of C and G.
Q. Read the following and answer the questions.
Today, 118 elements are known, the first 94 of which occur in nature. Of the 94 natural elements, eighty are stable.The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Elements are placed in the periodic table by their electron configurations, which exhibit periodic recurrences that explain the trends of properties across the periodic table. As we go across a period from left to right, we add a proton to the nucleus and an electron to the valence shell with each successive element. As we go down the elements in a group, the number of electrons in the valence shell remains constant, but the principal quantum number increases by one each time. An understanding of the electronic structure of the elements allows us to examine some of the properties that govern their chemical behavior. These properties vary periodically as the electronic structure of the elements changes.They are
(1) size (radius) of atoms and ions,
(2) ionization energies, and
(3) electron affinities.
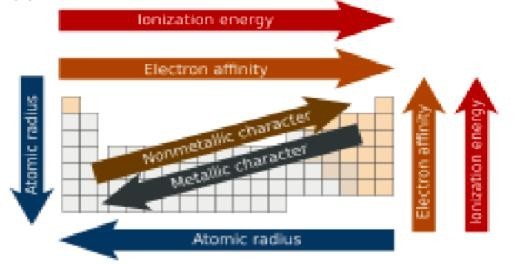
1. Which of the following set of elements is written in order of their increasing metallic character?
(a) Na, Li, K
(b) C, O, N
(c) Mg, Al, Si
(d) Be, Mg, Ca
2. What happens to tendency to gain electron in a period?
(a) Increases,
(b) Decreases,
(c) Remaining same,
(d) First increases then decreases.
3. Which of the following elements would lose an electron easily?
(a) Mg
(b) Na
(c) K
(d) Ca
4. Atomic size decreases from left to right in a period because
(a) Effective nuclear charge increases
(b) Number of shells remains the same
(c) Force of attraction between the nucleus and valence electrons increases
(d) All of these
Answer:
1 (d) 2 (d) 3 (c) 4 (d)
Chapter – 8
How do Organisms Reproduce?
Q. Read the paragraph and answer the questions.
The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their population. The process of sexual maturation for reproduction is gradual and takes place while general body growth is still going on. Some degree of sexual maturation does not necessarily mean that the mind or body is ready for sexual acts or for having and bringing up children. Various contraceptive devices are being used by human beings to control the size of population.
1) What are common signs of sexual maturation in boys
a) Broadening of shoulders
b) Development of mammary glands
c) Broadening of waist
d) High pitch of voice
2) Common sign of sexual maturation in girls is
a) Low pitch voice
b) Appearance of moustaches and beard
c) Development of mammary glands
d) Broadening of shoulders
3) Which contraceptive method changes the hormonal balance of the body a) Condoms
b) Diaphragms c) Oral pills
d) Both a) and b)
4) What should be maintained for healthy society
a) Rate of birth and death rate
b) Male and female sex ratio
c) Child sex ratio
d) None of these
Answer:
1) a) Broadening of shoulder
2) c) Development of mammary glands
3) c) Oral pills
4) b) Male and female sex ratio
Q. Read the following and answer the questions:
Preeti is very fond of gardening. She has different flowering plants in her garden. One day few naughty children entered her garden and plucked many leaves of Bryophyllum plant and threw them here and there in the garden. After few days, Preeti observed that new Bryophyllum plants were coming out from the leaves which fell on the ground.
1. What does the incidence sited in the paragraph indicate?
(a) Bryophyllum leaves have special buds that germinate to give rise to new plant.
(b) Bryophyllum an propagate vegetatively through leaves.
(c) Bryophyllum is a flowering plant that reproduces only asexually
(d) Both (a) and (b).
2. Which of the following plants can propagate vegetatively through leaves like Bryophyllum?
(a) Guava
(b) Begonia
(c) Ginger
(d) Mint
3. Do you think any other vegetative part of Bryophyllum can help in propagation? If yes, then which part?
(a) Roots
(b) Stems
(c) Flowers
(d) Fruits
4. Which of the following plant is artificially propagated (vegetatively) by stem cuttings in horticultural practices?
(a) Potato
(b) Snake plant
(c) Rose
(d) Water hyacinth
Answer:
1. (d)
2. (b)
3. (b)
4. (c)
Chapter – 9
Heredity and Evolution
Q. Read the following and answer questions from (i) to (iv)
Pea plants can have smooth seeds or wrinkled seeds. One of the phenotypes is completely dominant over the other. A farmer decides to pollinate one flower of a plant with smooth seeds using pollen from plant with wrinkled seeds. The resulting pea pod has all smooth seeds.
1) Which of the following conclusions can be drawn?
(i) The allele for smooth seeds is dominated over that of wrinkled seeds. (ii) The plant with smooth seeds is heterozygous.
(iii) The plant with wrinkled seeds is homozygous. (a) 1 only
(b) 1 and 2 only
(c) 1 and 3 only
(d) 1, 2 and 3
2) Which of the following crosses will give smooth and wrinkled seeds in same proportion?
(a) RR X rr
(b) Rr X rr
(c) RRX Rr
(d) rr X rr
3) On crossing of two heterozygous smooth seeded plants (Rr), a total of 1000 plants were obtained in F1 generation. What will be the respective number of smooth and wrinkled seeds obtained in F1 generation
(a) 750, 250
(b) 500, 500
(c) 800, 200
(d) 950, 50
4) The characters which appear in the first filial generation are called
(a) recessive characters
(b) dominant characters
(c) lethal character
(d) non-mendelian characters.
Answer:
1. (c) 1 and 3 only
3. (b) Rr x rr
4. (a) 750, 250
5. (b) dominant characters
Q. Read the passage and answer the given five questions
Pea plant is also tiny, easy to grow, and produces a big number of offspring. Pea plants can have tall plants or dwarf plants. One of the phenotypes is completely dominant over the other. A farmer decides to pollinate one flower of a tall plant with using pollen from plant with dwarf plant. The resulting pea pod has all tall plants.
1. Which of the following conclusions can be drawn?
(i) The allele for tallness is dominated over that of dwarfness.
(ii) The plant with tallness is heterozygous.
(iii) The plant with dwarfness is homozygous.
A. (i) only B. (i) and (ii) only C. (i) and (iii) only D. (i), (ii) and (iii).
2. Which of the following crosses will give tall and dwarf plants in same proportion?
A. TT x tt
B. Tt x tt
C. TT x Tt
D.tt x tt
3. Which of the following cross can be used to determine the genotype of a plant with dominant phenotype?
A. TTXTT
B. Tt x Tt
C. Tt x TT
D. Tt x tt
4. State the ratio of tall plant to dwarf plants in F2 genertion.
A. 2:2,
B.1:3
C.3:1,
D.4:0
Answer:
1. D. (i), (ii) and (iii).
2. B. Tt x tt
3. D. Ttxtt
4. C. 3:1
Chapter – 12
Electricity
Q. Read the paragraph and answer the questions.
Electrical resistivities of some substances, at 20°C are given below in the table. Study the table and answer the given questions.
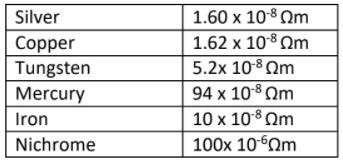
1. Which is a better conductor of electric current ?
(A) Silver
(B) Copper
(C) Tungsten
(D) Mercury
2. Which element will be used for electrical transmission lines ?
(A) Iron
(B) Copper
(C) Tungsten
(D) mercury U
3. Nichrome is used in the heating elements of electric heating device because:
(A) It has high resistivity
(B) It does not oxidise readily at high temperature
(C) Both of the above
(D) None of the above U
4. Series arrangement is not used for domestic circuits because:
(A) Current drawn is less (B) Current drawn is more (C) Neither of the above (D) Both of the above U
Answer.
1. (A)
2. (B)
3. (C)
4. (A)
Q. Read the paragraph and answer the questions.
In the given circuit, three identical bulbs B1, B2 and B3 are connected in parallel with a battery of 4.5 V. Study the diagram and answer the questions given below :
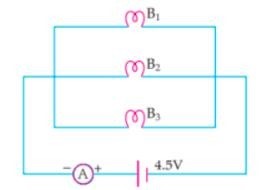
1. What will happen to the other two bulbs if the bulb B3 gets fused ?
(A) They will also stop glowing.
(B) Other bulbs will glow with same brightness.
(C) They will glow with low brightness.
(D) They glow with more brightness.
2.If the wattage of each bulb is 1.5 W, how much readings will the ammeter A
show when all the three bulbs glow simultaneously?
(A) 1.0 A
(B) 2 A
(C) 1.5 A
(D) None of the above
3. Find the total resistance of the circuit.
(A) 1.0 Ω
(B) 4.5 Ω
(C) 1.5 Ω
(D) 2.0 Ω
4. How many resistors of 88 Ω are connected in parallel to carry 10 A current on a220 V line ?
(A) 2 resistors (B) 1 resistors (C) 3 resistors (D) 4 resistors
Answer:
1. (B)
2. (A)
3. (B)
4. (D)
Chapter – 13
Magnetic Effects of Current
Q. Read the paragraph and answer the questions.
An electric motor is an electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a wire winding to generate force in the form of torque applied on the motor's shaft.
1) Electric motor is used in
(a) electric fans
(b) refrigerators
(c) mixers
(d) all of the above
2) In Electric motor magnetic field is produced by
(a) Permanent magnet
(b) Electro magnet
(c) both (a) and (b)
(d) none of the above.
3) Direction of magnetic force on a current carrying conductor placed in magnetic field is given by
(a) Fleming’s left hand rule (b) Fleming’s right hand rule (c ) Right hand palm rule
(d) none of the above
4) Moving part of an electric motor is called
(a)brushes
(b) shaft
(c) split ring
(d) slip ring
Answer:
1. (a)
2. (c)
3. (a)
4. (b)
Comments
Post a Comment